Type A and Type B Reactions: What They Are and Why They Matter
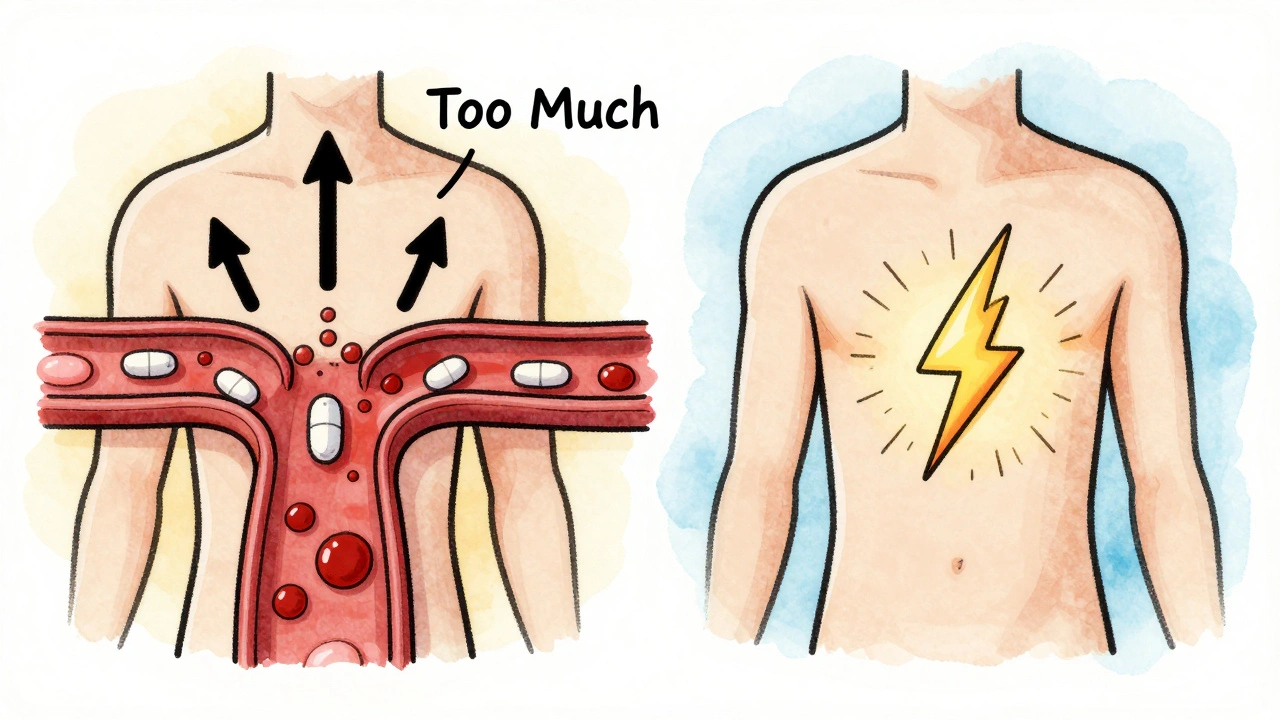
When a medication causes harm, it’s not always the same kind of problem. Type A reactions, predictable, dose-dependent side effects caused by a drug’s known pharmacology. Also known as augmented reactions, they’re the most common type of adverse drug reaction and often show up as extensions of the drug’s intended effect. Think of them like taking too much of a good thing—too much blood thinner leads to bleeding, too much insulin drops blood sugar too low. These reactions are avoidable, often tied to dosing errors, drug interactions, or patient factors like kidney or liver problems.
Type B reactions, unpredictable, idiosyncratic responses not related to the drug’s normal action. Also known as bizarre reactions, they’re rare but can be life-threatening. These aren’t about dose—they’re about your body’s unique reaction. A penicillin allergy? That’s Type B. A sudden, severe skin rash from a drug you’ve taken before without issue? Also Type B. They’re often immune-mediated, genetic, or triggered by unknown factors. Unlike Type A, you can’t predict them by reading the label. That’s why reporting even rare side effects through systems like MedWatch, the FDA’s official platform for tracking adverse drug events matters so much.
The difference between these two types shapes how we use drugs safely. Type A reactions show up in clinical trials and warning labels—they’re the reason you’re told not to drink alcohol with certain meds, or why your doctor checks your kidney function before prescribing. Type B reactions? They’re the ones that slip through. That’s why post-market monitoring, like analyzing reports from OpenFDA, a public tool for accessing FDA drug safety data, is critical. You might never hear about a Type B reaction until someone else reports it.
Many of the posts here tie directly into these concepts. You’ll find deep dives into how statins cause muscle pain (a Type A reaction tied to CoQ10 depletion), how tetracycline leads to sunburns (a predictable photosensitivity reaction), and why SGLT2 inhibitors raise yeast infection risk (a common, dose-related side effect). But you’ll also see stories about rare but serious reactions—like tramadol triggering seizures in some people, or generic drugs causing unexpected side effects due to inactive ingredients. Those are often Type B. Even the discussion around manufacturing defects in generics connects here: a capping issue might not change the active ingredient, but it could alter how the drug is absorbed, turning a safe Type A reaction into something unpredictable.
Understanding this split isn’t just for doctors. If you’ve ever wondered why a drug that works for your friend gave you a rash, or why your doctor keeps asking about every pill you take—even the ones you think are harmless—you now have the framework. Type A reactions are about what the drug does to your body. Type B reactions are about what your body does to the drug. One is preventable with care. The other needs vigilance—and reporting.
Dose-Related vs Non-Dose-Related Side Effects: What You Need to Know in Pharmacology
- Laura Ledas
- Dec, 1 2025
Learn the critical difference between dose-related and non-dose-related side effects in pharmacology. Understand why some reactions are predictable and others aren't - and what it means for your safety on medication.
Learn More