Tag: bioequivalence criteria
Bioequivalence Studies: What the FDA Requires Manufacturers to Prove
- Laura Ledas
- Feb, 15 2026
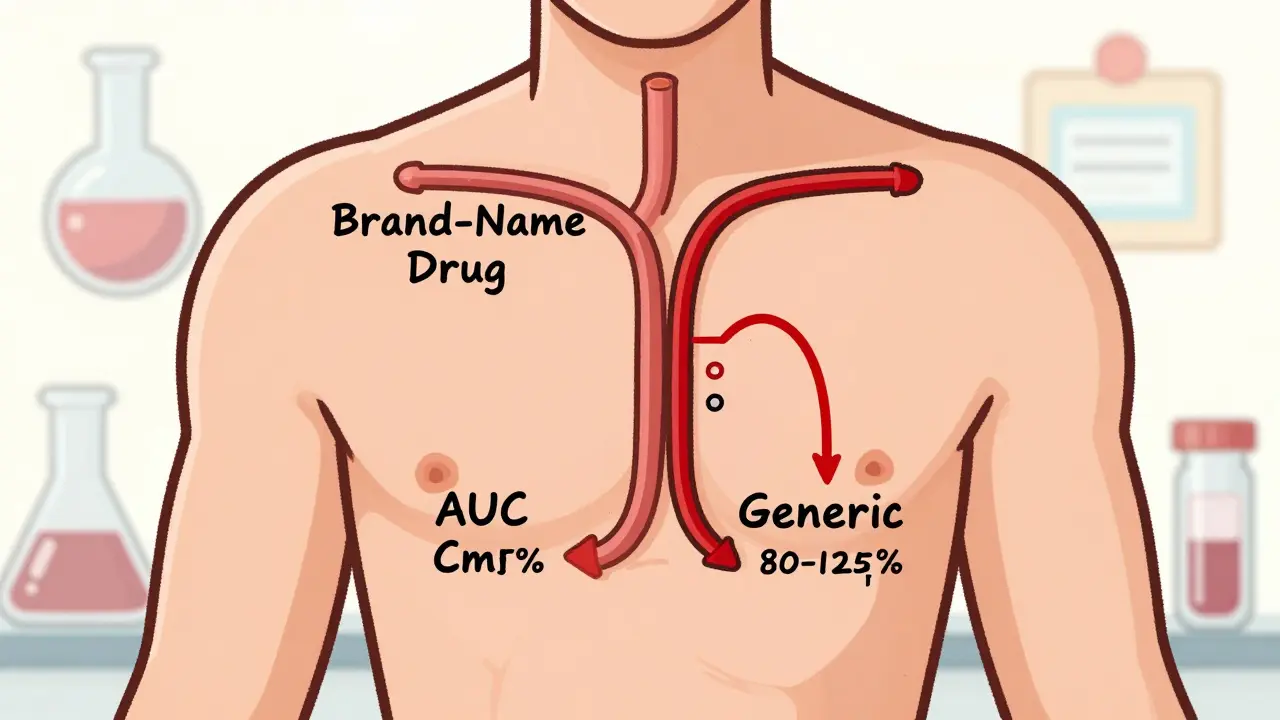
The FDA requires generic drug manufacturers to prove bioequivalence through rigorous clinical studies that show their product matches the brand-name drug in absorption and blood concentration. This ensures safety, effectiveness, and therapeutic equivalence.
Learn More