Every year, thousands of medication errors happen because someone misread an NDC number. It’s not a typo. It’s not a slip of the hand. It’s often a simple mistake in reading the three segments of a 10-digit code that looks like this: 00002-3105-01. One wrong digit can mean giving a patient a 40mg pill instead of a 20mg one - or worse, the wrong drug entirely. If you work in a pharmacy, hospital, or clinic, reading the NDC correctly isn’t optional. It’s your last line of defense against a preventable error.
What Exactly Is an NDC Number?
The National Drug Code, or NDC, is a unique 10-digit identifier assigned by the U.S. Food and Drug Administration (FDA) to every prescription and over-the-counter medication sold in the United States. Think of it like a barcode for drugs - but instead of scanning it, you have to read it. Every NDC breaks down into three parts: the labeler code, the product code, and the package code. Together, they tell you exactly which company made the drug, what the drug is, and how it’s packaged.
For example, the NDC 00002-3105-01 means:
- 00002 - The labeler code for Eli Lilly (the maker of Prozac)
- 3105 - The product code for 10mg capsules
- 01 - The package code for a bottle of 100 capsules
But here’s the catch: the NDC on the bottle might be printed as 5-3-2, 4-4-2, or 5-4-1. The format changes depending on the manufacturer. That’s why you can’t just glance at it. You have to count the digits between the hyphens to know what you’re looking at.
How to Break Down the Three Segments
Let’s go step by step. The first thing you do when you pick up a bottle is locate the NDC. It’s usually printed on the side or bottom of the container, often near the lot number and expiration date. Once you see it, pause. Don’t assume. Count the digits.
Segment 1: Labeler Code (Manufacturer)
This is the first part, and it’s 4 to 6 digits long. It tells you who made the drug - whether it’s Pfizer, Teva, or a small repackager. The FDA assigns these codes, and there are about 3,500 active ones. If the prescription says “Prozac from Eli Lilly,” but the NDC labeler code is 00185 (that’s Teva), you’ve got a problem. Maybe it’s a generic substitution - but you need to confirm that with the prescriber or the patient’s profile.
Segment 2: Product Code (Drug, Strength, Form)
This is the most critical part. It’s 3 or 4 digits and defines the actual medication: active ingredient, strength, and dosage form. For example:
- 3105 = Prozac 10mg capsule
- 4465 = Prozac 20mg capsule
- 1234 = Lisinopril 10mg tablet
- 5678 = Lisinopril 20mg tablet
Notice how changing just one digit changes the drug. A 2023 survey by the American Pharmacists Association found that 42% of all NDC-related errors happened because someone confused the product code - mistaking a 20mg tablet for a 10mg one, or a tablet for a liquid. That’s why you never skip this step. Always compare the product code to the prescription.
Segment 3: Package Code (Quantity and Type)
This is the last part - 1 or 2 digits. It tells you the size of the container: 01 = 100 capsules, 02 = 30 tablets, 10 = 500ml bottle. This doesn’t change the drug itself, but it matters for billing and inventory. If the prescription asks for 30 tablets but the NDC package code is 01 (100 tablets), you might be giving too much. Or worse - you might think you’re giving the right amount when you’re not.
Why the 11-Digit Format Matters
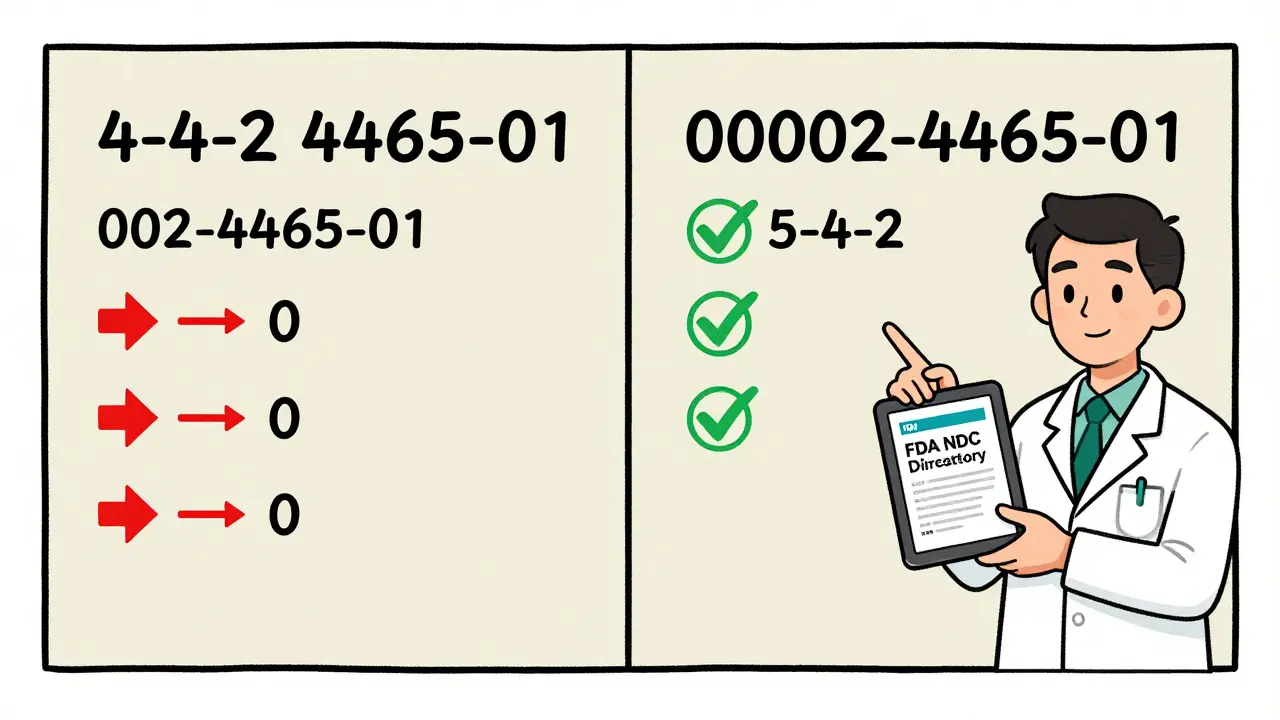
Here’s where things get messy. The NDC on the bottle is 10 digits. But when you bill Medicare, Medicaid, or private insurance, you must use an 11-digit version in the 5-4-2 format. That means you have to add a zero somewhere - but where?
It depends on the original format:
- If it’s 4-4-2 → becomes 5-4-2 by adding a zero at the start of the labeler code
- If it’s 5-3-2 → becomes 5-4-2 by adding a zero in the product code
- If it’s 5-4-1 → becomes 5-4-2 by adding a zero at the end of the package code
Example: 00002-3105-01 is already 5-4-2. No change needed. But 002-4465-01 (3-4-2) becomes 00002-4465-01 - you add two zeros to the labeler code to make it 5 digits. Mess this up, and your claim gets rejected. And if you’re using an electronic system that auto-converts, you still need to double-check. Systems make mistakes too.
How to Verify It for Safety
Don’t just read the NDC. Verify it. Here’s the process that saves lives:
- Check the prescription - Write down the drug name, strength, form, and quantity.
- Find the NDC on the bottle - Look for the hyphenated code.
- Identify the format - Count the digits between hyphens.
- Break it into segments - Labeler, product, package.
- Match each segment - Does the labeler match the brand? Does the product code match the strength and form? Does the package size match the quantity ordered?
- Convert to 11-digit format - If you’re billing, do this step carefully.
- Cross-check with the FDA database - Use the free NDC Directory on the FDA website. Type in the 10-digit code. It will show you the official drug name, manufacturer, and status. If it says “discontinued,” don’t dispense it.
One pharmacist in Arizona caught a fatal error when a patient’s prescription said “Lisinopril 5mg,” but the NDC on the bottle was for 20mg. The product code was close - 1234 vs. 1235 - but the difference was enough to cause a dangerous drop in blood pressure. She caught it because she verbalized each segment out loud during the double-check.
Common Mistakes and How to Avoid Them
Here are the top three errors - and how to stop them:
- Mixing up product and package codes - The product code tells you what the drug is. The package code tells you how many pills are in the bottle. Don’t confuse them. If the product code says “3105” (10mg capsule), don’t assume the package code “01” means 10mg. It means 100 capsules.
- Ignoring format changes - If you’re used to 5-4-2 and suddenly see a 5-4-1, don’t assume it’s the same. The last digit is different. That’s not a typo - it’s a different package size.
- Not checking discontinued codes - The FDA deactivates about 8,500 NDCs each year. A drug might still be in stock, but it’s no longer approved. Always verify the status in the FDA’s NDC Directory before dispensing.
Pro tip: Use the FDA’s mobile NDC Directory app. It’s free, updated daily, and works offline. You can scan the barcode or type in the code. It’ll tell you if it’s active, what the drug is, and who makes it. No guesswork.
When NDC Isn’t Enough
Here’s the hard truth: the NDC doesn’t tell you everything. Two drugs can have the same active ingredient - say, metformin - but different inactive ingredients. One might be gluten-free. Another might have dyes that cause allergic reactions. The NDC doesn’t reflect that. So if a patient has allergies or dietary restrictions, always check the full drug monograph or consult the pharmacist.
Also, generic drugs from different manufacturers can have the same NDC if they’re identical in formulation. But if they’re not - and sometimes they’re not - you need to know the brand. That’s why some hospitals require the brand name to be written on the prescription, not just the generic.
And don’t forget: the NDC doesn’t verify the patient. It doesn’t know if the right person got the right drug. That’s why the final step - checking the patient’s name, date of birth, and prescription - is just as important as reading the NDC.
What’s Changing in 2025?
The FDA is phasing out the current 10-digit formats and moving to a standardized 12-digit NDC by 2025. This means no more 4-4-2 or 5-3-2. Everything will be 6-4-2. It’s meant to reduce confusion. But until then, you still need to know the old system. Training programs for pharmacy techs now include both formats. If you’re new to this, learn the 10-digit system first - it’s still everywhere.
Final Checklist for NDC Verification
Before you hand over any medication, run through this:
- ✅ Did I locate the NDC on the packaging?
- ✅ Did I count the digits between hyphens to identify the format?
- ✅ Did I match the labeler code to the expected manufacturer?
- ✅ Did I confirm the product code matches the drug, strength, and form on the prescription?
- ✅ Did I check that the package code matches the quantity ordered?
- ✅ Did I convert to 11-digit format if billing?
- ✅ Did I verify the NDC in the FDA’s NDC Directory?
- ✅ Did I confirm the patient’s identity and allergies?
If you can answer yes to all eight, you’ve done your job. Medication errors don’t happen because people are careless. They happen because people skip steps. Don’t be one of them.
What does the NDC number stand for?
NDC stands for National Drug Code. It’s a unique 10-digit identifier assigned by the U.S. FDA to every prescription and over-the-counter medication sold in the United States. It helps identify the manufacturer, drug formulation, strength, and package size.
Can two different drugs have the same NDC number?
No. Each NDC number is unique to a specific drug product - meaning the same manufacturer, active ingredient, strength, dosage form, and package size. If any of those change, even slightly, the NDC changes too. However, different manufacturers may produce identical generic drugs with different NDCs.
Why do I need to convert the NDC to 11 digits?
Medicare, Medicaid, and most insurance systems require billing claims to use an 11-digit NDC in the 5-4-2 format. The 10-digit code on the bottle may be in a different format (like 4-4-2 or 5-3-2), so you must add a zero to the appropriate segment to meet the billing standard. Failing to do so can lead to claim denials.
How do I know if an NDC is still active?
Use the FDA’s free National Drug Code Directory online. Enter the 10-digit NDC, and it will show whether the product is active, discontinued, or listed as unavailable. Never dispense a medication with a discontinued NDC, even if it’s still in stock.
Can I rely on the barcode scanner to read the NDC correctly?
Barcode scanners are helpful, but they’re not foolproof. They can misread damaged labels, smudged print, or similar-looking digits (like 0 and O, or 1 and I). Always visually verify the NDC against the prescription and the FDA database - even if the scanner says it’s correct.
What should I do if the NDC doesn’t match the prescription?
Stop. Do not dispense. Contact the prescribing provider or pharmacist to confirm the correct medication. Document the discrepancy. Never assume it’s a labeling error - it could be a dangerous mix-up. The NDC is your safety net. If it doesn’t match, treat it as a red flag until proven otherwise.
Is the NDC the same as the lot number?
No. The NDC identifies the drug product (manufacturer, strength, form, package). The lot number identifies a specific batch of that product for tracking in case of recalls. You need both, but they serve different purposes.
Do over-the-counter (OTC) drugs have NDC numbers?
Yes. All OTC medications sold in the U.S. must have an NDC, just like prescription drugs. This includes pain relievers, antacids, allergy meds, and cough syrups. Always verify the NDC on OTC products if they’re being dispensed by a pharmacy or used in a clinical setting.

11 Responses
Been working ER pharmacy for 12 years. Saw a guy get a 40mg Zoloft instead of 20mg because someone skimmed the product code. He ended up in ICU. NDC isn’t just paperwork-it’s the difference between going home and going in a body bag.
Always read it out loud. Even if you’re tired. Even if the line’s long. Say it. Out. Loud.
My grandma’s meds used to come in these weird little bottles with codes that looked like alien math. I used to help her check ‘em. Now I’m a tech. Turns out that little hyphen between 3105 and 01? That’s her life right there.
Don’t rush it. Breathe. Count. Verify. It’s not busywork. It’s love in action.
USA makes the best drugs. NDC system works. Stop overcomplicating it. Just read the damn numbers.
From India, but I train pharmacy staff here. This is gold. We don’t have NDC here, but the concept is universal. Always verify. Always double-check. One digit can kill. Simple as that.
While the NDC system is robust, its reliance on manual digit interpretation remains a systemic vulnerability. The transition to 12-digit standardization is not merely administrative-it is a necessary evolution in patient safety protocol.
Man, this post made my day. I’ve been a pharmacy tech for 3 years and I still get nervous every time I pick up a new bottle. But now I know I’m not alone in caring. Keep doing good work, everyone. You’re saving lives one digit at a time 💪❤️
Oh wow. A whole 2000-word essay on how to read numbers. Did we forget that we have computers now? Or is this just a glorified TED Talk for people who think ‘automation’ is a dirty word?
Let me guess-you also hand-wash your stethoscopes and still use paper logs? Cute. The FDA’s system is a relic. But hey, at least you’re thorough. I’ll be over here letting AI handle it while you count hyphens like it’s 1999.
Is the 11-digit billing format standardized across all payers? Or do some still use 10-digit? I’ve seen claims get denied for both formats and I’m not sure if it’s a system error or my conversion.
Let’s be real-this is basic competency. If you need a 1500-word guide to read an NDC, you shouldn’t be handling medications. The FDA’s NDC Directory is publicly accessible, the format is documented, and the consequences of error are catastrophic. This isn’t ‘pro tip’ material. It’s job 101.
And yes, I’ve seen the ‘I thought 01 meant 10mg’ mistake. It’s not a typo. It’s negligence dressed up as ‘busy.’
Carol, I hear you. Techs are drowning in apps, alerts, and EHR pop-ups. But here’s the thing: the human eye still catches what the scanner misses. That smudged ‘0’ that looks like ‘O’? That’s your patient’s life.
I used to think automation was the answer. Then I saw a barcode scan a 50mg oxycodone as 5mg. The system didn’t flag it. The tech didn’t look. Kid almost died.
Don’t let tech make you lazy. Use it. But never trust it blindly. Stay sharp. Stay slow. Stay human. 🙏
UK doesn’t use NDC. We use BNF codes. But the principle’s the same. Read it. Check it. Don’t guess. I’ve seen people confuse ‘100mg’ with ‘10mg’ on a label. Same result: chaos.